Computer System Validation Services
Let us take the Validation Overhead and you focus on the business…Our experts can ensure your computer system validation (CSV) complies with GAMP5, ASTM-E2500, ISO, ICH and other industry standards.
- Manufacturing Equipment/ Software - DCS, PLC, SCADA, BMS, MES
- Laboratory Equipment/ Software - LIMS
- Enterprise Applications - Veeva Vault, ERP, CTMS
- IT Infrastructure - Data Center, Network, Servers
- Cloud Validation (IaaS, PaaS, SaaS) - AWS, Azure
Continuous Compliance/ Validation Solution
Are you ready to take IT teams away from responding reactively to audit requests?
Continuous compliance is about developing a culture and strategy within the organization that continually assesses the compliance status to ensure you are meeting your industry and regulatory demands while maintaining secure systems.
In short, we help you to take IT teams away from responding reactively to audit requests and be ready by being proactively prepared for the future threats.
Our advanced technology platform specifically designed for your environment will help you to achieve the below Target State:
- Compliance through continuous monitoring.
- No more point in time Validation/qualification
- No more paper reports
- Review only by exception
- Single pane of glass for end-to-end Validation, Qualification and SOX
Consulting Services
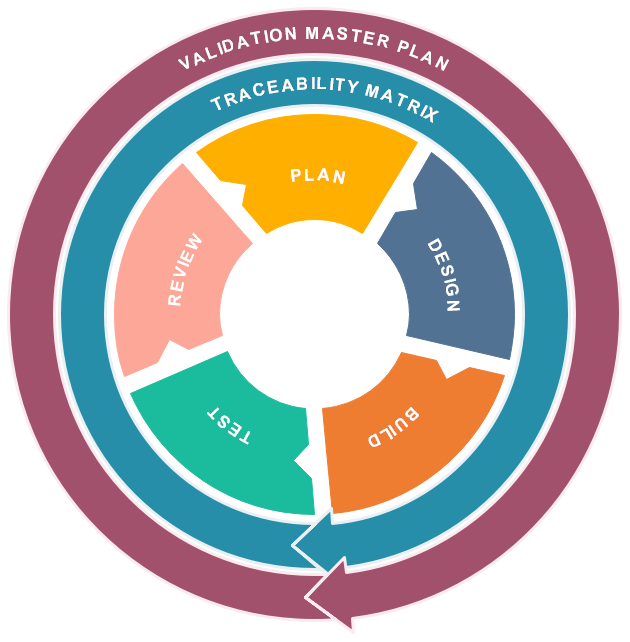
Consulting Solution for every phase of the Validation Life Cycle.
Our team of validation experts will work closely with your QA and IT teams, as well as software vendors to ensure that a coherent validation strategy is implemented in line with regulatory requirements, organization standards and associated risks with the system.
Computer Software Assurance (CSA)
Our knowledgeable consultants can help you proactively transition from CSV to CSA. A few key best practices for facilitating the transition from CSV to CSA methodology include,
- Perform a validation assessment.
- Develop a transition plan
- Embrace the change
- Audit vendors
- Embark on digitization and automation
- Develop a change management plan
Computer System Validation
RxCloud adopts a life cycle approach to validation that enables you to start, scale and maintain your operations.
Our GxP Computer System Validation consultants are immersed in the regulations and guidelines set forth by the FDA, EMA and other regulatory bodies including
- 21 CFR Part 11
- Annex 11
- ISO 9001:2015
- ASTM E2500
- GAMP5
Starting from assessments, planning and execution out subject matter experts set you up for success no matter where you are in the life cycle.


